Cells are constantly sensing and adapting to their environment. In response to an external signal, they have the ability to "polarize" themselves by reorganizing their internal skeleton, the cytoskeleton, and repositioning their organelles along an axis defined by the position of the signal. The design of an artificial cell able to polarize itself from elementary components represents an interesting strategy to improve our understanding of the principles of self-organization of living organisms and could open the way to the elaboration of materials with capacities of adaptation to a stimulus.
Polarity acquisition is a primitive function that allows unicellular cells to move towards a nutrient source or to escape from a predator. Within a tissue, it allows cells to orient their secretory, absorptive, or signaling activities according to the position and shape of neighboring cells. Although the molecular actors vary from one organism to another, the mechanisms involved in polarization seem to be conserved in living organisms. They are based on the reorganization of the cytoskeleton. In animal cells, the highly dynamic actin cytoskeleton is the first to react by locally adjusting its organization to the signal. The microtubule network then adapts to the multiple local structures of the actin network. The radial organization of the microtubules around the organizing center, the centrosome, allows it to integrate its information at the scale of the whole cell and to define a single global response. The mechanism of information integration is still unknown. It involves a repositioning of the centrosome towards the signal in response to a reorganization of forces in the microtubule network. Where and how are the forces generated on the microtubules? How are they integrated at the centrosome? How does the actin network influence these forces? These are all questions that need to be addressed to understand the mechanisms involved in cell polarity.
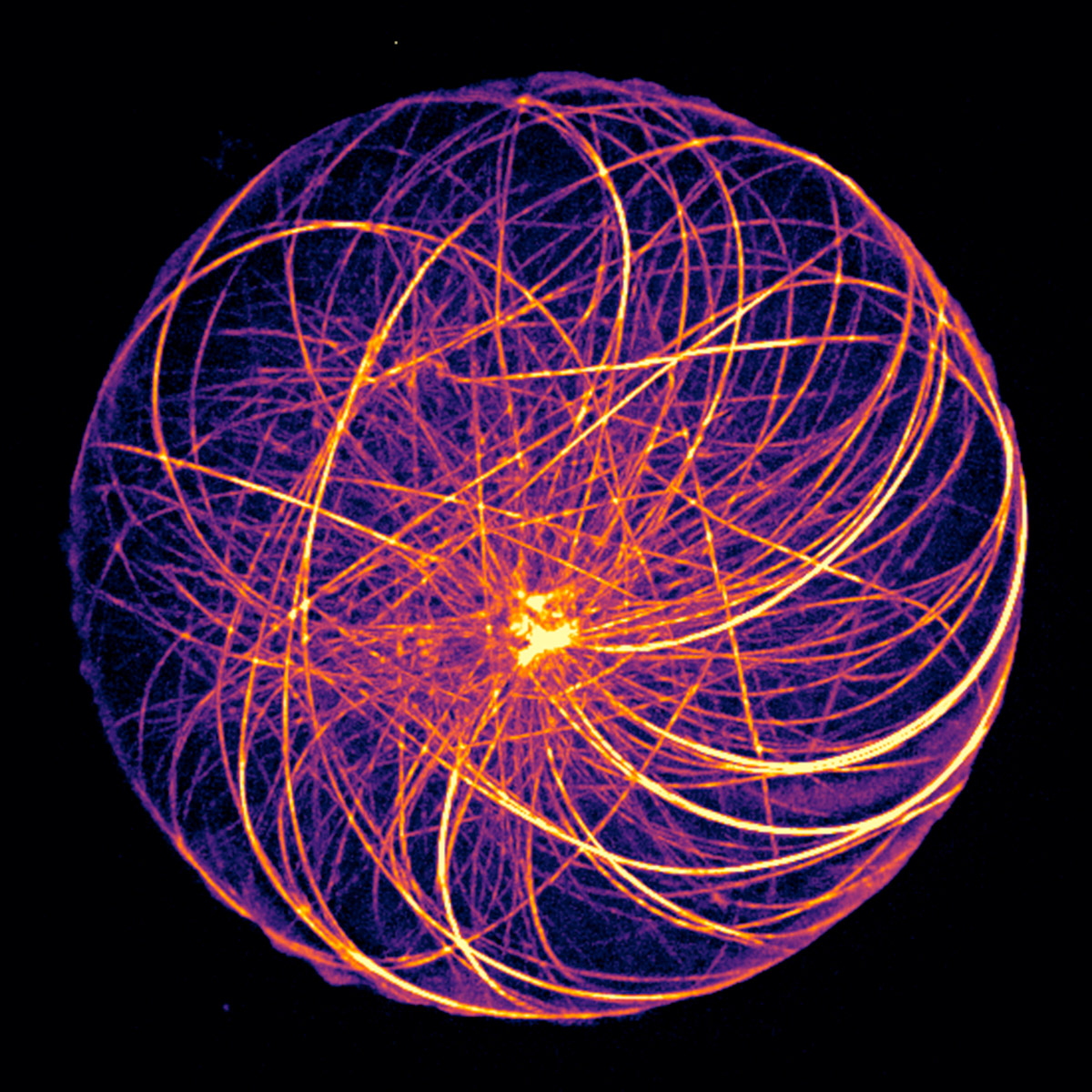
Organization of microtubules in a cell-sized compartment (diameter 60 µm).
Crédit : Jérémie Gaillard, Alfredo Sciortino, Benoît Vianay, Cytomorpholab.
Researchers at the
CytomorphoLab team used purified proteins to reconstruct
in vitro in cell-sized microwells (Image above), the interaction of a microtubule aster with actin networks of various architectures. In the absence of actin filaments, the positioning of the aster is very sensitive to variations in MT length. Actin networks limit the sensitivity of MTOC positioning to MT length and reinforce the centering or decentering of MTOCs according to the isotropy of their architecture.
These results show that actin networks can impose constraints on microtubules allowing the control of MTOC positioning according to MT length. Moreover, the actin network enhances the centering or decentering of the MTOC depending on its architecture. The results and the techniques implemented represent an important step towards the reconstitution in an artificial cell of one of the primitive functions of living organisms: polarity acquisition.