In eukaryotic cells, each membrane has a specific lipid composition (or lipid profile). The general question investigated by the team is to decipher the molecular processes responsible for the establishment and maintenance of glycer
olipid profiles, in membranes and lipid droplets, in plastid-containing eukaryotes (plants and algae).
Glycerolipids are made by the assembly of fatty acids (FAs), glycerol and polar heads. Less than a dozen classes of glycerolipids are sufficient to compose most biological membranes, including
phospholipids such as phosphatidylcholine (PC), phosphatidylethanolamine (PE), the major constituents of extra-plastidial membranes, and
non-phosphorous galactolipids, such as monogalactosyldiacylglycerol (MGDG) or digalactosyldiacylglycerol (DGDG), which form the bulk of photosynthetic membranes (Figure 1). In algae, a third class of
non-phosphorous membrane glycerolipids, called the betaine lipids, is localized in extraplastidial membranes.
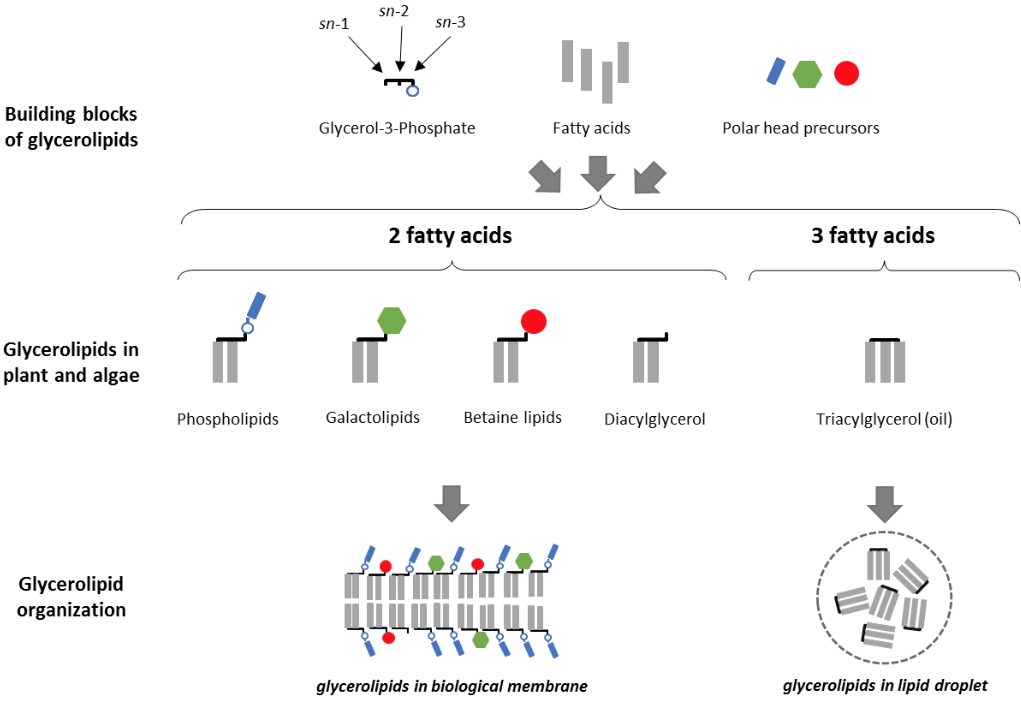 Figure 1: Glycerolipid, constituents of biological membranes and oils. Glycerolipids are a specific group of lipids, consisting of a glycerol backbone to which 1 to 3 fatty acids are linked by an ester bond. Glycerolipids containing two fatty acids may have a polar head, and are used to build bilayers, forming the matrix of biological membranes. Glycerolipids containing three fatty acids are called triacylglycerols, or oils, and accumulate in the form of droplets within the cells.
Figure 1: Glycerolipid, constituents of biological membranes and oils. Glycerolipids are a specific group of lipids, consisting of a glycerol backbone to which 1 to 3 fatty acids are linked by an ester bond. Glycerolipids containing two fatty acids may have a polar head, and are used to build bilayers, forming the matrix of biological membranes. Glycerolipids containing three fatty acids are called triacylglycerols, or oils, and accumulate in the form of droplets within the cells.
In plants, the biosynthesis of glycerolipids schematically follows 4 rules:
- The biosynthesis of fatty acids takes place in the stroma of the chloroplast;
- The assembly of fatty acids and glycerol to form most of the phospholipids takes place in the endoplasmic reticulum (ER);
- The synthesis of galactolipids, sulfolipid and some of the phosphatidylglycerol takes place in the plastid envelope;
- The membrane glycerolipids can eventually be converted into triacylglycerol (TAG), a reserve lipid stored in lipid droplets.
Consequently, the lipid composition of a membrane depends on 3 major phenomena: (1) the neosynthesis of glycerolipids; (2) the conversion between glycerolipids, through the exchange of fatty acids or polar heads and (3) the glycerolipid transport/trafficking within the cell. The proportions in lipid classes in each membrane are very stable. In physiological terms, these steady states correspond to a complex series of processes called “membrane lipid homeostasis”, which provides the framework for the research lines in the team.
Our work is in line with the past research of the team,
i.e.
advancing knowledge of the processes setting up the membrane lipid distribution in plastid-containing eukaryotes at steady state (membrane lipid homeostasis) and tuning this steady state in response to environmental changes (lipid remodeling). The overall strategy remains
to consider the glycerolipid metabolic scheme as a system, and
to contribute to the international effort to characterize its components and dynamics by a multidisciplinary approach. Our questions will focus on eukaryotes containing plastid derived from primary or secondary endosymbiosis.
Our team has a solid knowledge in the field of plant glycerolipids.
In plants, the enzymes of lipid synthesis and modification are well known. In microalgae, the knowledge base is much weaker and requires investigation of all the bases of lipid metabolism. Microalgae include organisms originating from primary endosymbiosis (Archaeplastida), like plants, but also from secondary endosymbiosis (Chromista).
Our models of primary endosymbiosis are the plant
Arabidopsis thaliana, the picoalga
Ostreococcus tauri, and Chlorophyta species developing in cold environments, including the snow algae
Sanguina nivaloides. As with other eukaryotes, lipid trafficking and sorting systems in plants are poorly understood, a knowledge gap that we are working to fill with the
A. thaliana model.
O.
tauri is a minimal plant system and a marine organism at the evolutionary crossroads of the two major microalgal kingdoms (Archaeplastida, Chromista). It is an ideal model for studying the gene networks underlying the reprogramming of marine microalgae-specific lipid metabolism in response to abiotic stresses.
Our secondary endosymbiosis models are the marine microalgae (
Phaeodactylum tricornutum,
Microchloropsis gaditana), which accumulate oil under stress conditions, (especially
M. gaditana) and for which modern genetic manipulation tools are developed (CRISPR-CAS9). Ice diatoms (
e.g. Fragilariopsis cylindrus) are also studies.
Finally, since membranes and reserve glycerolipids are the first to respond to thermal variations, and in particular to cold, the team is paying particular attention to organisms growing in snow and ice.
Our aims are to:
1) characterize lipid trafficking and flow between organelles;
2) decipher the role and control of lipid homeostasis at the membrane, cell and organism levels;
3) reconstruct the pathways of membrane lipid synthesis;
4) establish the relationships among lipid biosynthesis, membrane expansion and cell architecture;
5) understand the link with the abiotic environment of the organisms, in particular the oligotrophic environments poor in nutrients, and the thermal variations, cold, snow, ice.
Our challenging scientific issues for the future include 1) the characterization of the lipid trafficking systems and fluxes, 2) the deciphering of the role and the control of glycerolipid homeostasis within the whole system, 3) the reconstruction of membrane lipid pathways and 4) the relationship between lipid biosynthesis and membrane expansion and architecture. Concerning the translation of knowledge for applied sciences, the control of TAG accumulation should be sought through the development of chemical genetics and synthetic biology approaches, in a rigorous collaborative context with industrial partners. To achieve these goals, the team will rely on the research projects of each P.I., the access to a unique lipidomics platform, the development of cutting-edge electron microscopy methods, and the existence of two project teams involved in industrial partnership. Four main research axes will drive our research (Figure 2):
- Homeostasis of glycerolipids in secondary endosymbionts;
- Lipid trafficking in photosynthetic primary endosymbionts;
- Remodeling of glycerolipids in microalgae and plants in response to abiotic parameters, including those characterizing alpine environments;
- Gene regulatory networks underlying the reprogramming of lipid metabolism in marine microalgae.

The team coordinates the
AlpAlga project on the biodiversity and physiology of algae in high altitude environments