For a long time, photo-autotrophic organisms have captured researchers' attention, as they serve as entry points for carbon in the life cycle and are the primary CO2 fixers in the living world. Researchers at LPCV/Photosynthesis have developed an innovative approach to photo-physiology at the unicellular and subcellular levels. It is crucial to unravel the mechanisms optimizing photosynthesis at the individual cell level since light undergoes various transformations as it penetrates different cellular layers and even organs. Moreover, within the tissue, cells can react differently depending on their developmental stage or physiology.
Researchers from LPCV, internationally renowned, have enhanced photosynthesis evaluation by combining a confocal microscope with saturating pulses to achieve the following three essential objectives:
- Investigate the specialization of photosynthetic activities within the developing tissues of non-vascular plants. For example, in the moss Physcomitrium patens, photosynthetic capacities at the chloroplast level are similar across different vegetative tissues, with observable differences at the macroscopic level attributed solely to chloroplast density variation.
- Identify a specific subpopulation of phytoplankton cells involved in marine photosymbiosis. Immediately after symbiosis establishment, symbiotic algae are photosynthetically inactive, in a quiescent state. After adaptation, they achieve better photosynthetic efficiency than free-living algae.
- Explore the link between light penetration and photoprotection responses within different tissues composing a plant leaf's anatomical structure.
This simple method can be adapted to various sample types, serving as a versatile tool for studying plant and microalgae photosynthetic acclimation and, consequently, for CO2 capture.
Fundings: Europenan Research Council, ERC Chloro-mito (grant n°833184) the European Union H2020 Project BIOTEC-02-2019 GAIN4CROPS (grant no 862087)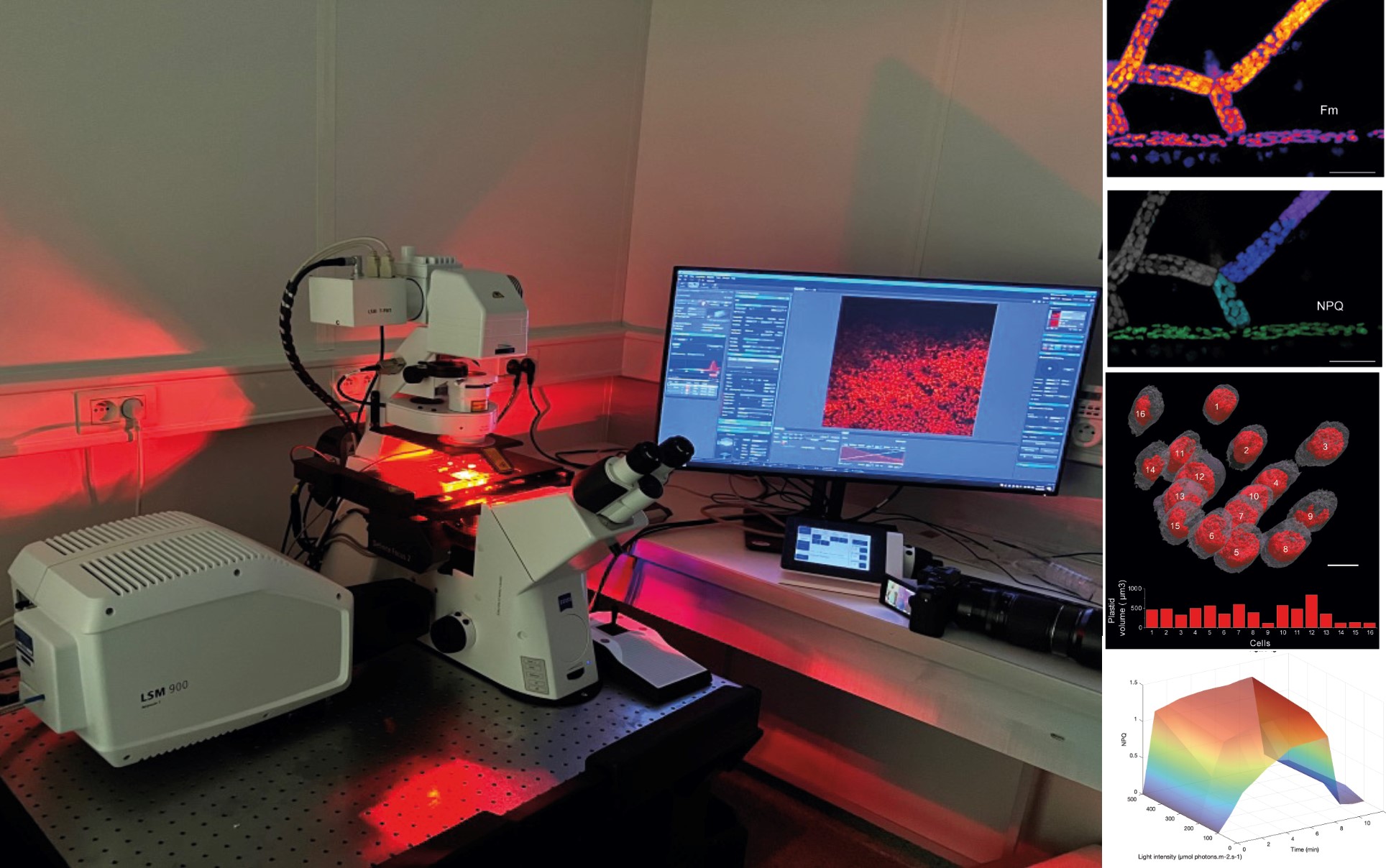
Figure: Overview of the confocal microscopy setup with its red light enabling photosynthesis activation. On the right: various types of achievable outcomes. Visualization of photosynthetic parameters directly on the microscopic image (Fmax and NPQ; top), 3D reconstruction of cells and analysis of their photosynthetic capacity (middle), representation of photosynthetic protection capacities based on time and light (bottom)