In order to improve oil production by microalgae for applications such as biofuel development, the choice of species is crucial. While some microalgae are well known and can be used to target genes, the study of other microalgae starts with a blank page. This is the case of the Heterokont group, a little-known branch of evolution in which we can find oleaginous microalgae that are very interesting because they can be grown industrially, such as
Microchloropsis gaditana. It is this microalgae that has been selected by researchers and engineers of the Irig within the framework of a partnership with TotalEnergies, a partnership initiated in 2014.
When the choice of microalgae species is made, the rational approach to increase oil production requires the selection of gene targets, increasing or decreasing their expression and/or function. As for the oil to be produced, it corresponds to a highly hydrophobic triacylglycerol (TAG) molecule. Inside the cell, TAG is sequestered in a spherical structure called an "oil droplet"; it is in equilibrium with other cellular glycerolipids, such as those found in biological membranes that contain only two fatty acids.
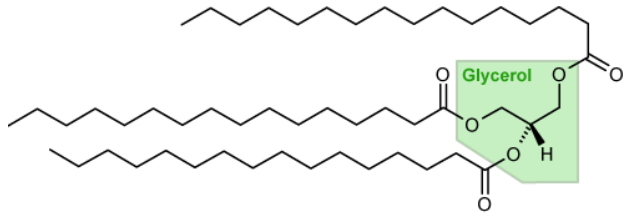
A triacylglycerol (TAG) molecule consists of a glycerol skeleton on which three fatty acids are esterified. Example of tripalmitoylglycerol. The general formula of TGA is:
CH
2-COO-R1
|
CH-COO-R2
|
CH
2-COO-R3
While studying
M. gaditana, the researchers noted that there was an enzyme called "
ACS Bubblegum", an
Acyl-CoA Synthetase (ACS) type never before described in photosynthetic organisms. By genomic editing using molecular scissors (TALEN method), it was not possible to completely abolish this gene in
M. gaditana, highlighting its vital role. However, several very fine modifications of the gene coding ACSBG have altered its function. In these mutants, a study of all membrane glycerolipids and TAG was performed on the LIPANG lipidomics platform of our laboratory. This study made it possible to characterize the precise role of ACSBG. This enzyme prepares fatty acids for their esterification on certain membrane lipids. Mutations introduced in the gene cause structural modifications that directly influence its activity (
Figure). These alterations in the synthesis of membrane lipids are then compensated by a reorientation of the fatty acids, which are found in excess, towards TAGs, resulting in a gain in oil productivity.
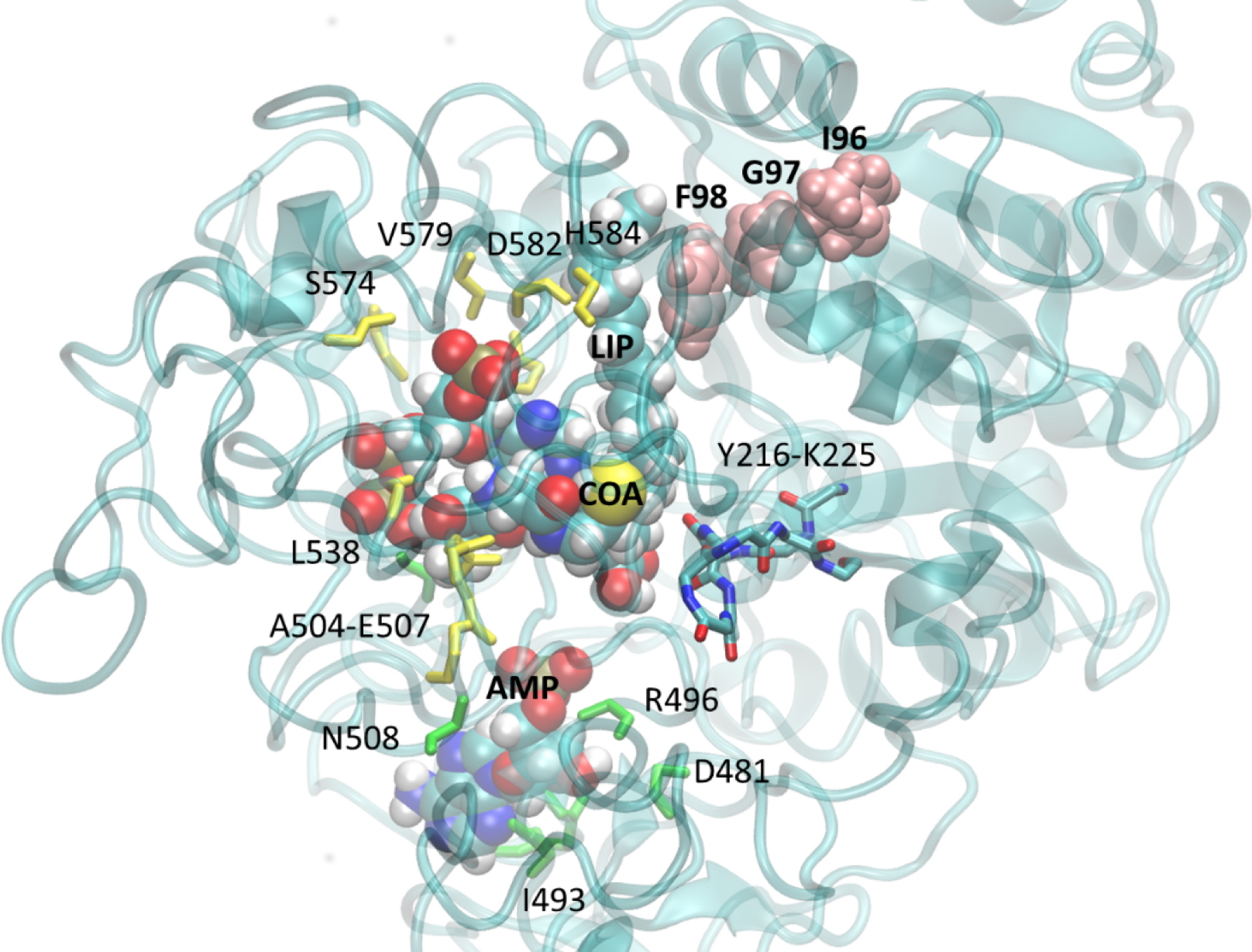
3D model of
M. gaditana's ACSBG protein, associated with its substrates, Coenzyme-A (CoA), Adenosine Monophosphate (AMP) and a fatty acid (LIP). The point mutations introduced by genetic editing using TALEN are found at the level of the amino acids "IGF" (in pink). These mutations modify the conformation of the protein at the catalytic site (identified by the numbered amino acids), and cause a decrease in enzymatic activity leading to a complete reorientation of fatty acid flows in the cell.
The ACS known as "ACS Bubblegum" (or ACSBG), takes its name from the effect of the corresponding mutation in the Drosophila fly, characterized by the appearance of bubble-like structures in the first optic ganglion, as well as a strong lipid imbalance.
It is possible to act on the enzymes that direct fatty acids to membrane lipids or TAGs. Fatty acids cannot be
esterified directly because the esterification reaction requires energy. They must first be coupled to a chemical group, for example Co-enzyme A (CoA), through a bond that "stores" the energy required for subsequent esterification. Fatty acids are thus "prepared", or energetically activated, by association with CoA by enzymes called
Acyl-CoA Synthetases (ACS).
This work was conducted in collaboration between the Lipid, the Photosynthesis, the Signal and the Flo_RE teams at LPCV and TotalEnergies. Serge Crouzy from the Chemistry and Biology of Metals laboratory of the Irig carried out the structural modeling of the enzyme. This work was the subject of a patent.