Mechanical forces exerted by cells play a crucial role on the proper functioning of organs by controlling notably their shape and development. At the microscopic level, each cell has an internal framework, the cytoskeleton, made up of specialized proteins that ensure the generation and transmission of cellular forces to their environment. Among these proteins, actin forms filaments that are reorganized by the action of molecular motors called myosins, generating contractile cables called stress fibers that resemble elastic springs stretched between two anchoring points. Although these structures are widely recognized as being involved in force generation at the molecular level, the mechanism by which forces are generated and distributed at the entire cell-level remains unknown.
In order to characterize the production of forces in these contractile cables, we cut them by making a precise incision using a laser beam (
Figure 1).
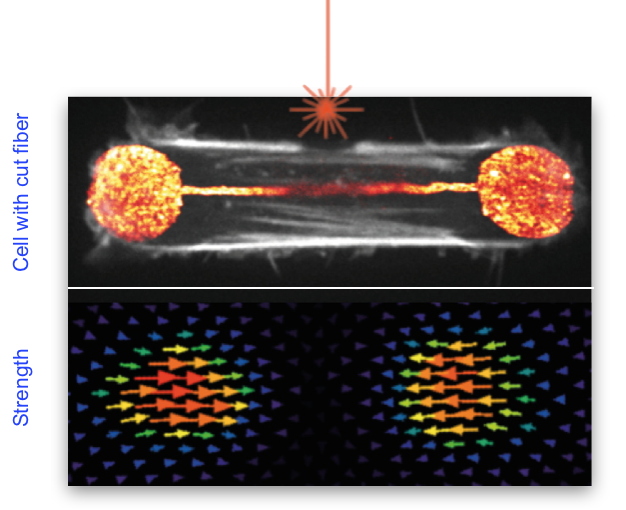
Figure 1: Illustration showing the cleavage of a stress fiber in a retinal epithelial cell (in white in the top image) using a laser (shown in orange above the fiber). The lower image represents the forces developed by this cell on its support.
This manoeuvre led to an unexpected result: the total cell force was barely affected, showing that other structures in the cytoskeleton were at work to generate and transmit force at the cell level. The same technique was then used to produce a cut in the actin network found along the cell membrane, a network previously considered as passive. By subsequently modeling the contribution of the entire actin network through physical parameters, we understood that the entire sub-membrane actin network was also contributing to force production and not only the stress fibers. Using several complementary techniques to improve the resolution of the cytoskeleton images, we also discovered that the stress fibers, far from being isolated structures, were in fact completely entangled in the surrounding actin network lining the membrane. Finally, we investigated the formation of stress fibers by making videos and thus revealed that they had an unexpected origin: the contraction and alignment of the fibers along the membrane, thus explaining their embedding in this network.
These results offered us the opportunity to propose a new model for the assembly of stress fibers, according to which the points where the cells are anchored to their external environment direct the tension in the actin network lining the membrane, which aligns and concentrates the filaments and induces the formation of stress fibers (
Figure 2). This study also revealed the existence of mechanical integrity within the cell which, although composed of multitudes of small filaments, behaves as a fully connected object.
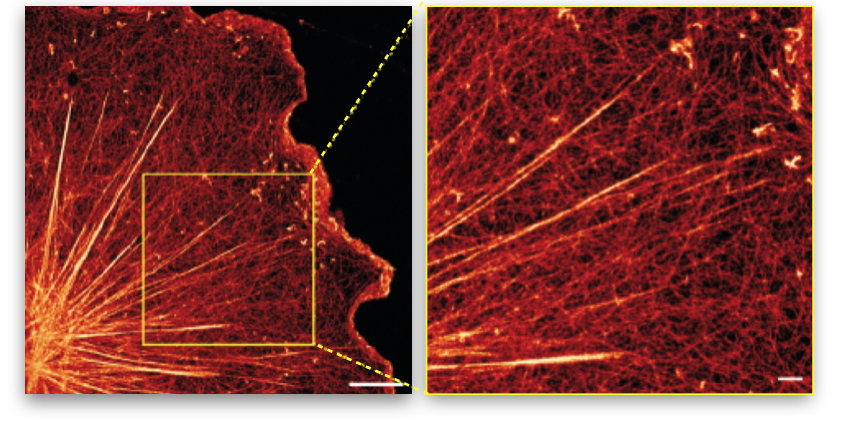
Figure 2: Illustration of stress fibers embedded in an actin membrane network.
Super-resolution image of the actin network of a glial cell (nervous system cell) showing the entanglement of large stress fibers with the surrounding membrane actin network (right image = zoom of the left image).