Uranium (U) is a harmful radionuclide that may be absorbed by plants from soil and, consequently, contaminate the food chain, with human health hazards. The cellular toxicity and detoxification mechanisms of U are still poorly described but likely result from its ability to bind strongly to biomolecules, mainly proteins. Thus, identification and characterization of U-binding proteins (UraBPs) in plants is an important step toward the elucidation of the mechanisms of plant response and adaptation to U stress at the molecular level.
We have developed, in collaboration with researchers from
IBS,
SyMMES,
BIAM and
DMTS, an accurate and reliable metalloproteomics approach to isolate and identify at a broad scale UraBPs from root and shoot tissues of the plant model
Arabidopsis thaliana, thus elucidating the first “urano-proteome” from a photosynthetic organism. One of the identified UraBPs of particular interest and hitherto identified as the plasma membrane-associated cation-binding protein 1 (PCaP1) was characterized in more detail, by using a combination of spectroscopic (metal-binding assays, tryptophan-fluorescence titrations), structural (solution-state NMR studies) and
in planta (reverse genetics) approaches.
We showed that purified recombinant Arabidopsis PCaP1 protein overproduced in bacteria was able to bind U
in vitro with high-affinity (in the nanomolar range), but also copper and oxidized iron in very high proportions (up to 60 iron cations per protein monomer), and that calcium competed with U for binding.
In addition, we showed that U induced PCaP1 oligomerization
in vitro, through binding at the monomer interface, at both the N-terminal structured domain and the C-terminal flexible region of the protein (
Figure). Finally, they observed that U transfer from roots to aerial parts of Arabidopsis plants was strongly affected in a
pcap1 null-mutant, suggesting a role for this protein in ion mobility
in planta.
These findings represent an important milestone to advance knowledge in the fields of radionuclide toxicology and phytoremediation (depollution of soils by plants).
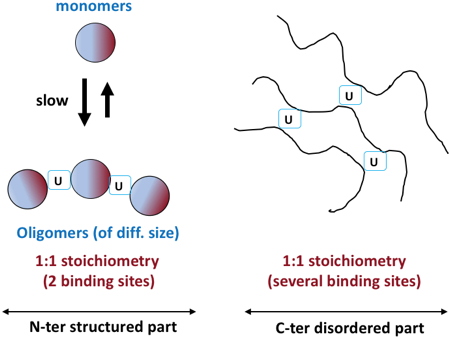
Schematic model for uranyl binding to the N-terminal structured and the C-terminal disordered parts of
Arabidopsis thaliana PCaP1 protein, based on spectroscopic and solution-state NMR studies.
IBS: Institut de biologie structurale
SyMMES: Molecular Systems and nanoMaterials for Energy and Health Laboratory
DMTS: Medicines and Healthcare Technologies Department
BIAM: Institute of Biosciences and Biotechnologies of Aix-Marseille